Materials Up Close & Personal: ACRYLIC
17 July 2020

This week’s blog leaves behind the ancient histories of cork, wax and salt, fast forwarding in time to explore a much more recent addition to our materials palette: ACRYLIC.
We rarely notice this naturally transparent, cheap, lightweight and shatter-proof substitute for glass, whose pseudonym – Perspex – derives from the Latin ‘to see through’. Whether we are aware of it or not though, we’re seeing (through) more and more of it at the moment, with acrylic ‘sneeze screens’ being installed in our shops, pharmacies and restaurants to enforce social distancing and cut down the transmission of infection. But acrylic isn’t just a barrier between us – it’s also responsible for Cardi B’s glorious nail art and David Hockney’s technicolour scenes of Californian suburbia, as well as pearly dentures and piano keys, hard contact lenses and soft floor waxes, car headlights and more.
Acrylic is a material of many names, otherwise known as Perspex, Plexiglas, Lucite, Acrylite, Polycast and Altuglas, to name but a few of its alter egos. These substances are all manufactured by different companies, each with their own unique recipe, production process, and trade name, but are all essentially variants of the same plastic made from a chemical called methacrylate. Poly(methyl methacrylate) - or PMMA for short, for us lazy materials enthusiasts - is the most common acrylic plastic. It was first developed in the early 1900s by Otto Rohm, a German chemist working in the leather and textiles industry, who dedicated his whole professional career to the search for useable plastic products derived from acrylic acids. In the 1930’s, after many years of research and development (and several explosions), a few different plastics companies, including Rohm’s, arrived at workable and economically viable forms of the acrylic we know and love today. Since then, the process for making acrylic has been refined, giving us a highly transparent thermoplastic that is relatively scratch resistant (for a clear plastic), very stable in UV light (it doesn’t yellow in sunlight like the celluloid that came before it), and although it’s quite brittle by comparison to more impact-resistant plastics like polycarbonate, much less likely to shatter than glass.

Because it is one of the clearest plastics we have, acrylic first found favour in the 1930s as a glass substitute in safety goggles, as well as World War 2 military applications like gas masks, submarine periscopes and cockpit windows. Because of this superb clarity, acrylic is also the material of choice for enclosures around exhibits in museums and galleries, including Damien Hirst’s array of unfortunate perspex-encapsulated and formaldehyde-pickled animals and our Materials Library sample of asbestos, which has been safely sealed inside an acrylic box by specialists to stop any of its hazardous inhalable hairs from escaping.
You probably also have acrylic to thank for your next endoscopy: PMMA is a cheaper, more flexible and more robust alternative to glass optical fibres, which comes in handy when we need to shine a light inside our bodily cavities to see what’s going on in there. Plastic optical fibres consist of an extremely thin extruded acrylic core that is clad in a layer of another (often fluorinated) plastic with a lower refractive index than the acrylic. This difference in the light reflecting and transmitting properties of the two plastics causes light that is travelling through the acrylic core to be reflected every time it hits the fluorinated plastic cladding, so that it bounces around inside the acrylic core, producing the phenomenon of total internal reflection. This means that light - and images of our internal organs – are efficiently transmitted down the length of the acrylic fibre, without any being lost out of the sides of the fibre on the way.

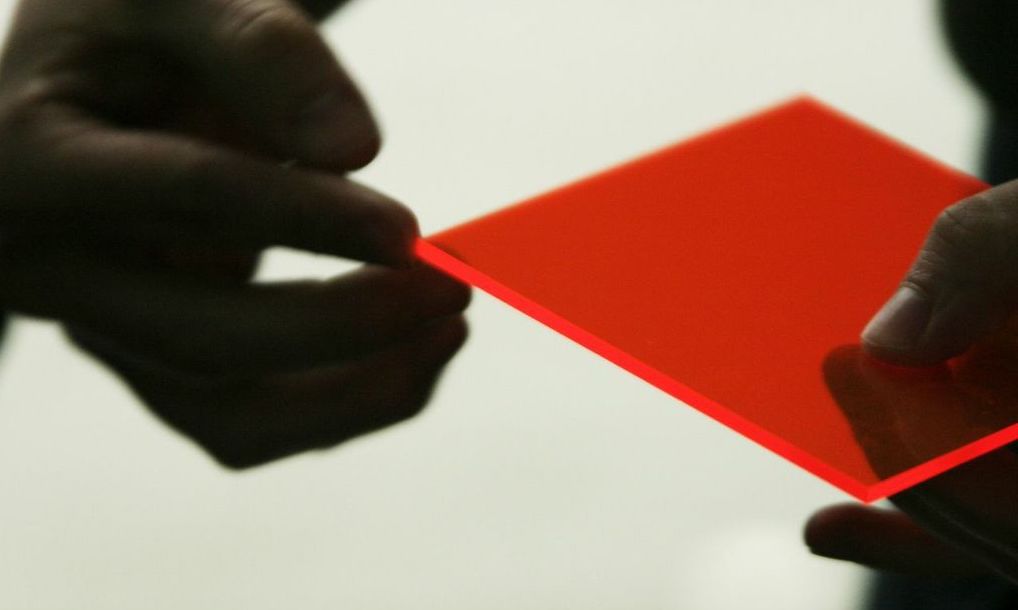
This phenomenon of internal refraction is also responsible for our Materials Library sheet of live-edged Perspex, which glows at the edges under ambient light as if its lit from inside. This happens because light is gathered by this sheet of acrylic on its flat surfaces, and is then reflected internally until it is emitted wherever that surface is interrupted – at the edges, or wherever there is a scratch or etched mark. The acrylic also contains fluorescent dyes to accentuate this effect, so the light emitted gets stronger under UV light.
As you can see from this dayglo sample, acrylic is a very talented and versatile purveyor of colour, available in pretty much every shade imaginable. This makes acrylic the perfect material for creating seriously colourful and glamourous contemporary nail art, where artificial nails are glued to the natural fingernail, acting as long, thin canvases for layers of acrylic powder and liquid, mixed with pigments, decorative Swarovski crystals, glitters and decals. Solid acrylic plastic (like all polymers) is made by chemically assembling a large number of small building blocks called monomers, so that they become long chains of molecules . This polymerisation process is what’s happening when the liquid acrylic in your nails ‘dries’. Depending on the type of acrylic system used, the liquid acrylic monomer is given the energy to assemble itself into long polymer chains by UV light, heat, or a chemical catalyst.
PMMA is also responsible for acrylic paint, which was received with a lot of excitement by artists when it was developed in the 1960s. The fluidity and radiance of this new, quick-drying synthetic paint brought with it a faster, looser way of working that was distinct from traditional, slow oil painting. One of the first acrylic paints, Magna, was made up of ground acrylic resin in a solvent meaning it could be still be thinned with turpentine and white spirit just like its oily precursor. This particularly smooth and tactile paint had a loyal following of artists like Roy Lichenstein, but the solvents and wetting agents in it were later found to be carcinogenic. Paint manufacturers quickly switched to making the water-based acrylic we use today: a combination of ultrafine solid acrylic powder, water and pigments, brought together to create a quick-drying solution that can be easily thinned with water. Once squeezed out of its sealed tube, the water in this colourful gloop evaporates, leaving behind resin particles that fuse together, trapping the pigment inside and forming a hard but flexible pigmented film.
Although the solvent in early acrylic paint was toxic, acrylic itself is actually quite well-suited to being implanted into our bodies. Although the first clinical application of PMMA was in 1930s, when it was used to repair cranial defects in monkeys, acrylic wasn’t used to repair human bodies until the 1940s. Its biocompatibility was discovered accidentally when World War 2 pilots whose eyes were injured by shards of broken acrylic from plane cockpits fared much better than those affected by shards of shattered glass. These tragic injuries led directly to the invention of the first prosthetic intraocular lens: a dome of acrylic that was implanted into the eye to replace the natural lens as part of cataract surgery. These rigid PMMA lenses, which were also worn on top of the eye as the first hard contact lenses, have mostly been replaced by more flexible lenses. These can be folded, so they don’t require such a big incision to implant them.
Acrylic was, and still is, used extensively in dentistry, where its similarity to human dentine has seen it take over from ivory, animal bone and human teeth as the material of choice for dentures, dental implants and dental crowns. Orthopaedic surgeons also caught on to acrylic’s advantages, developing PMMA ‘bone cement’: a kind of space-filling plastic ‘grout’ that sits between our bones and an artificial knee or hip joint, absorbing shock and distributing pressure. Acrylic’s ability to encapsulate and carry other substances has come in handy here too: it can be impregnated with active substances like antibiotics so it can deliver drugs directly to the site of the surgery.
Acrylic is also used as a cheaper and more ethical replacement for the elephant and hippo tusks (or walrus and sperm whale teeth) that were traditionally used to make piano keys. Modern-day ‘ivories’ in all but the most expensive pianos are actually made from plastic, but ‘tickling the acrylics’ doesn’t have quite same ring to it! Using acrylic in piano keys brings all sorts of benefits, including less illegal poaching of elephants, and keys that don’t get dirty and yellowed over time. However, some pianists still hanker for the particular tactile and ‘responsive’ qualities of ivory, so manufacturers have been trying to develop more realistic synthetic substitutes. Yamaha’s high-end pianos, for example, use their proprietary ‘Ivorite’ material, which is thought to look and feel more like ivory, but is actually a composite of ABS plastic and mineral fillers.
This lucid material is not perfect, and its main downsides are its propensity to stress-crack, and its poor recyclability. Acrylic is a thermoplastic, which means that unlike a thermoset plastic, it can be re-formed again and again using heat, which allows it to be easily reworked by casting, extrusion and injection moulding. However, this propensity to being reformed into new shapes shouldn’t be confused with its ability to be depolymerised (broken down into its constituent monomer building blocks), in order to be rebuilt into new, high-quality plastic. The most common way to recycle PMMA is using a process called pyrolysis, which involves heating it to 450° in the absence of oxygen. Many recycling centres don’t have the equipment or expertise to pyrolise acrylic, so it often ends up in landfill or incineration. Because acrylic is used in a lot of LCD screens, smart phones and solar cells, however, there’s a lot of promising research currently going on to find better ways to recycle it.
About our blog series 'Materials: Up Close & Personal'
We are publishing a new series of ten extra-long Materials Library profiles, through which our Materials Librarian Sarah Wilkes will pay homage to those silent and humble materials that we surround ourselves with daily, but that often we don’t even notice. Every week or so, she will be exploring the expansive inner lives and backstories of the surfaces, substances and stuff around us, and sharing it with our community through our blog and social media #MaterialsLibraryUpCloseAndPersonal.
We would love you to join in too! Send us a picture of one of your favourite household substances with a few words about what you’ve noticed after spending some time getting to know your material cohabitants. We will periodically post a collection of these little gems on our website.